42 9.7 Neutralization and Titration Practice
Key Concepts and Summary
The stoichiometry of chemical reactions may serve as the basis for quantitative chemical analysis methods. Titrations involve measuring the volume of a titrant solution required to completely react with a sample solution. This volume is then used to calculate the concentration of analyte in the sample using the stoichiometry of the titration reaction.
Chemistry End of Chapter Exercises
- What volume of 0.0105-M HBr solution is required to titrate 125 mL of a 0.0100-M Ca(OH)2 solution?
[latex]\text{Ca(OH)}_2(aq) + 2\text{HBr}(aq) \longrightarrow \text{CaBr}_2(aq) + 2\text{H}_2 \text{O}(l)[/latex] - Titration of a 20.0-mL sample of acid rain required 1.7 mL of 0.0811 M NaOH to reach the end point. If we assume that the acidity of the rain is due to the presence of sulfuric acid, what was the concentration of sulfuric acid in this sample of rain?
- What is the concentration of NaCl in a solution if titration of 15.00 mL of the solution with 0.2503 M AgNO3 requires 20.22 mL of the AgNO3 solution to reach the end point?
[latex]\text{AgNO}_3(aq) + \text{NaCl}(aq) \longrightarrow \text{AgCl}(s) + \text{NaNO}_3(aq)[/latex] - In a common medical laboratory determination of the concentration of free chloride ion in blood serum, a serum sample is titrated with a Hg(NO3)2 solution.
[latex]2\text{Cl}^{-}(aq) + \text{Hg(NO}_3)_2(aq) \longrightarrow {2\text{NO}_3}^{-}(aq) + \text{HgCl}_2(s)[/latex]What is the Cl− concentration in a 0.25-mL sample of normal serum that requires 1.46 mL of 8.25 × 10−4M Hg(NO3)2(aq) to reach the end point?
- Potatoes can be peeled commercially by soaking them in a 3-M to 6-M solution of sodium hydroxide, then removing the loosened skins by spraying them with water. Does a sodium hydroxide solution have a suitable concentration if titration of 12.00 mL of the solution requires 30.6 mL of 1.65 M HCl to reach the end point?
- What volume of 0.600 M HCl is required to react completely with 2.50 g of sodium hydrogen carbonate?
[latex]\text{NaHCO}_3(aq) + \text{HCl}(aq) \longrightarrow \text{NaCl}(aq) + \text{CO}_2(g) + \text{H}_2 \text{O}(l)[/latex] - What volume of 0.08892 M HNO3 is required to react completely with 0.2352 g of potassium hydrogen phosphate?
[latex]2\text{HNO}_3(aq) + \text{K}_2 \text{HPO}_4(aq) \longrightarrow \text{H}_2 \text{PO}_4(aq) + 2\text{KNO}_3(aq)[/latex] - What volume of a 0.3300-M solution of sodium hydroxide would be required to titrate 15.00 mL of 0.1500 M oxalic acid?
[latex]\text{C}_2 \text{O}_4 \text{H}_2(aq) + 2\text{NaOH}(aq) \longrightarrow \text{Na}_2 \text{C}_2 \text{O}_4(aq) + 2\text{H}_2 \text{O}(l)[/latex] - What volume of a 0.00945-M solution of potassium hydroxide would be required to titrate 50.00 mL of a sample of acid rain with a H2SO4 concentration of 1.23 × 10−4M.
[latex]\text{H}_2 \text{SO}_4(aq) + 2\text{KOH}(aq) \longrightarrow \text{K}_2\text{SO}_4(aq) + 2\text{H}_2 \text{O}(l)[/latex] - A sample of solid calcium hydroxide, Ca(OH)2, is allowed to stand in water until a saturated solution is formed. A titration of 75.00 mL of this solution with 5.00 × 10−2M HCl requires 36.6 mL of the acid to reach the end point.
[latex]\text{Ca(OH)}_2(aq) + 2\text{HCl}(aq) \longrightarrow \text{CaCl}_2(aq) + 2\text{H}_2 \text{O}(l)[/latex]What is the molarity?
- What mass of Ca(OH)2 will react with 25.0 g of propionic acid to form the preservative calcium propionate according to the equation?
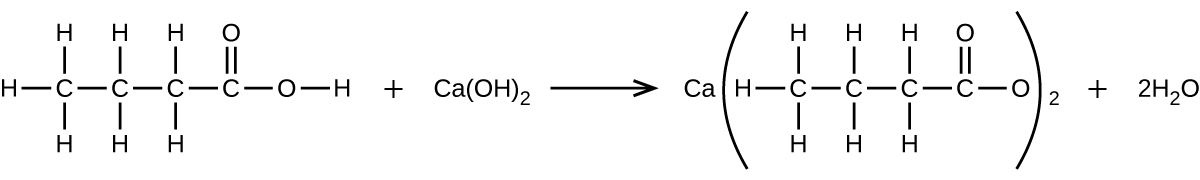
- How many milliliters of a 0.1500-M solution of KOH will be required to titrate 40.00 mL of a 0.0656-M solution of H3PO4?
[latex]\text{H}_3\text{PO}_4(aq) + 2\text{KOH}(aq) \longrightarrow \text{K}_2 \text{HPO}_4(aq) + 2\text{H}_2 \text{O}(l)[/latex] - Potassium acid phthalate, KHC6H4O4, or KHP, is used in many laboratories, including general chemistry laboratories, to standardize solutions of base. KHP is one of only a few stable solid acids that can be dried by warming and weighed. A 0.3420-g sample of KHC6H4O4 reacts with 35.73 mL of a NaOH solution in a titration. What is the molar concentration of the NaOH?
[latex]\text{KHC}_6 \text{H}_4 \text{O}_4 (aq) + \text{NaOH}(aq) \longrightarrow \text{KNaC}_6 \text{H}_4 \text{O}_4(aq) + \text{H}_2 \text{O}(aq)[/latex] - The reaction of WCl6 with Al at ~400 °C gives black crystals of a compound containing only tungsten and chlorine. A sample of this compound, when reduced with hydrogen, gives 0.2232 g of tungsten metal and hydrogen chloride, which is absorbed in water. Titration of the hydrochloric acid thus produced requires 46.2 mL of 0.1051 M NaOH to reach the end point. What is the empirical formula of the black tungsten chloride?
Glossary
- analyte
- chemical species of interest
- buret
- device used for the precise delivery of variable liquid volumes, such as in a titration analysis
- combustion analysis
- gravimetric technique used to determine the elemental composition of a compound via the collection and weighing of its gaseous combustion products
- end point
- measured volume of titrant solution that yields the change in sample solution appearance or other property expected for stoichiometric equivalence (see equivalence point)
- equivalence point
- volume of titrant solution required to react completely with the analyte in a titration analysis; provides a stoichiometric amount of titrant for the sample’s analyte according to the titration reaction
- gravimetric analysis
- quantitative chemical analysis method involving the separation of an analyte from a sample by a physical or chemical process and subsequent mass measurements of the analyte, reaction product, and/or sample
- indicator
- substance added to the sample in a titration analysis to permit visual detection of the end point
- quantitative analysis
- the determination of the amount or concentration of a substance in a sample
- titrant
- solution containing a known concentration of substance that will react with the analyte in a titration analysis
- titration analysis
- quantitative chemical analysis method that involves measuring the volume of a reactant solution required to completely react with the analyte in a sample
Solutions
Answers to Chemistry End of Chapter Exercises
2. 3.4 × 10−3M H2SO4
4. 9.6 × 10−3M Cl−
6. 49.6 mL
8. 13.64 mL
10. 1.22 M
12. 34.99 mL KOH
14. The empirical formula is WCl4.

