36 9.1 Molarity
Learning Objectives
By the end of this section, you will be able to:
- Describe the fundamental properties of solutions
- Calculate solution concentrations using molarity
- Perform dilution calculations using the dilution equation
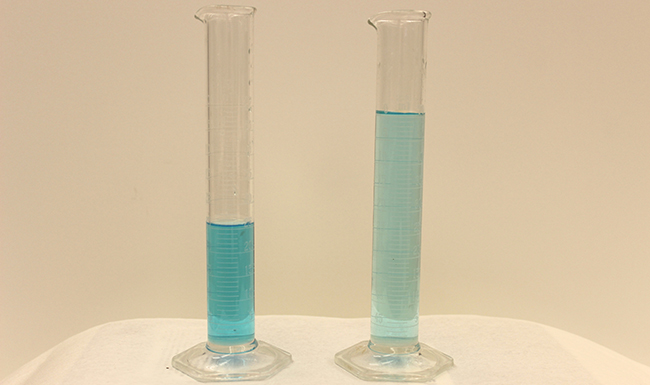
In preceding sections, we focused on the composition of substances: samples of matter that contain only one type of element or compound. However, mixtures—samples of matter containing two or more substances physically combined—are more commonly encountered in nature than are pure substances. Similar to a pure substance, the relative composition of a mixture plays an important role in determining its properties. The relative amount of oxygen in a planet’s atmosphere determines its ability to sustain aerobic life. The relative amounts of iron, carbon, nickel, and other elements in steel (a mixture known as an “alloy”) determine its physical strength and resistance to corrosion. The relative amount of the active ingredient in a medicine determines its effectiveness in achieving the desired pharmacological effect. The relative amount of sugar in a beverage determines its sweetness (see Figure 1). In this section, we will describe one of the most common ways in which the relative compositions of mixtures may be quantified.

Solutions
We have previously defined solutions as homogeneous mixtures, meaning that the composition of the mixture (and therefore its properties) is uniform throughout its entire volume. Solutions occur frequently in nature and have also been implemented in many forms of manmade technology. We will explore a more thorough treatment of solution properties in the chapter on solutions and colloids, but here we will introduce some of the basic properties of solutions.
The relative amount of a given solution component is known as its concentration. Often, though not always, a solution contains one component with a concentration that is significantly greater than that of all other components. This component is called the solvent and may be viewed as the medium in which the other components are dispersed, or dissolved. Solutions in which water is the solvent are, of course, very common on our planet. A solution in which water is the solvent is called an aqueous solution.
A solute is a component of a solution that is typically present at a much lower concentration than the solvent. Solute concentrations are often described with qualitative terms such as dilute (of relatively low concentration) and concentrated (of relatively high concentration).
Concentrations may be quantitatively assessed using a wide variety of measurement units, each convenient for particular applications. Molarity (M) is a useful concentration unit for many applications in chemistry. Molarity is defined as the number of moles of solute in exactly 1 liter (1 L) of the solution:
Example 1
Calculating Molar Concentrations
A 355-mL soft drink sample contains 0.133 mol of sucrose (table sugar). What is the molar concentration of sucrose in the beverage?
Solution
Since the molar amount of solute and the volume of solution are both given, the molarity can be calculated using the definition of molarity. Per this definition, the solution volume must be converted from mL to L:
Check Your Learning
A teaspoon of table sugar contains about 0.01 mol sucrose. What is the molarity of sucrose if a teaspoon of sugar has been dissolved in a cup of tea with a volume of 200 mL?
Answer:
0.05 M
Example 2
Deriving Moles and Volumes from Molar Concentrations
How much sugar (mol) is contained in a modest sip (~10 mL) of the soft drink from Example 1?
Solution
In this case, we can rearrange the definition of molarity to isolate the quantity sought, moles of sugar. We then substitute the value for molarity that we derived in Example 1, 0.375 M:
[latex]0.375\;M = \frac{\text{0.375 mol solute}}{\text{1 L solution}}[/latex]
Rearrange the molarity equation to solve for mol of solute:
[latex]\text{mol solute} = M \times{\text{L solution}}[/latex]
Plug back in and calculate (make sure your units cancel out):
[latex]\text{mol solute} =\frac{\text{0.375 mol sugar}}{\text{1 L}} \times (10 \;\text{mL} \times \frac{1 \text{L}}{1000 \;\text{mL}}) = 0.004 \;\text{mol sugar}[/latex]
Check Your Learning
What volume (mL) of the sweetened tea described in Example 1 contains the same amount of sugar (mol) as 10 mL of the soft drink in this example?
Answer:
80 mL
Example 3
Calculating Molar Concentrations from the Mass of Solute
Distilled white vinegar (Figure 2) is a solution of acetic acid, CH3CO2H, in water. A 0.500-L vinegar solution contains 25.2 g of acetic acid. What is the concentration of the acetic acid solution in units of molarity?

Solution
As in previous textbox shaded, the definition of molarity is the primary equation used to calculate the quantity sought. In this case, the mass of solute is provided instead of its molar amount, so we must use the solute’s molar mass to obtain the amount of solute in moles:
Check Your Learning
Calculate the molarity of 6.52 g of CoCl2 (128.9 g/mol) dissolved in an aqueous solution with a total volume of 75.0 mL.
Answer:
0.674 M
Example 4
Determining the Mass of Solute in a Given Volume of Solution
How many grams of NaCl are contained in 0.250 L of a 5.30-M solution?
Solution
The volume and molarity of the solution are specified, so the amount (mol) of solute is easily computed as demonstrated in Example 2:
[latex]\text{mol solute} = \frac{\text{5.30 mol NaCl}}{\text{1 L}} \times 0.250 \;\text{L} = 1.325 \;\text{mol NaCl}\\[0.5em][/latex]
Finally, this molar amount is used to derive the mass of NaCl:
[latex]1.325 \;\text{mol NaCl} \times \frac{58.44 \;\text{g NaCl}}{\text{mol NaCl}} = 77.4 \;\text{g NaCl}[/latex]
Check Your Learning
How many grams of CaCl2 (110.98 g/mol) are contained in 250.0 mL of a 0.200-M solution of calcium chloride?
Answer:
5.55 g CaCl2
When performing calculations stepwise, as in Example 4, it is important to refrain from rounding any intermediate calculation results, which can lead to rounding errors in the final result. In Example 4, the molar amount of NaCl computed in the first step, 1.325 mol, would be properly rounded to 1.32 mol if it were to be reported; however, although the last digit (5) is not significant, it must be retained as a guard digit in the intermediate calculation. If we had not retained this guard digit, the final calculation for the mass of NaCl would have been 77.1 g, a difference of 0.3 g.
In addition to retaining a guard digit for intermediate calculations, we can also avoid rounding errors by performing computations in a single step (see Example 5). This eliminates intermediate steps so that only the final result is rounded.
Example 5
Determining the Volume of Solution Containing a Given Mass of Solute
In Example 3, we found the typical concentration of vinegar to be 0.839 M. What volume of vinegar contains 75.6 g of acetic acid?
Solution
First, use the molar mass to calculate moles of acetic acid from the given mass:
Then, use the molarity of the solution to calculate the volume of solution containing this molar amount of solute:
Combining these two steps into one yields:
[latex]\text{g solute} \times \frac{\text{mol solute}}{\text{g solute}} \times \frac{\text{L solution}}{\text{mol solute}} = \text{L solution}[/latex]
Now plug in the numbers:
[latex]75.6 \;\text{g CH}_3\text{CO}_2\text{H} (\frac{\text{1 mol CH}_3\text{CO}_2\text{H}}{60.05 \;\text{g}}) (\frac{\text{1 L solution}}{0.839 \;\text{mol CH}_3\text{CO}_2\text{H}}) = 1.50 \;\text{L solution}[/latex]
Check Your Learning
What volume of a 1.50-M KBr solution contains 66.0 g KBr?
Answer:
0.370 L
Dilution of Solutions
Dilution is the process whereby the concentration of a solution is lessened by the addition of solvent. For example, we might say that a glass of iced tea becomes increasingly diluted as the ice melts. The water from the melting ice increases the volume of the solvent (water) and the overall volume of the solution (iced tea), thereby reducing the relative concentrations of the solutes that give the beverage its taste (Figure 3).
Dilution is also a common means of preparing solutions of a desired concentration. By adding solvent to a measured portion of a more concentrated stock solution, we can achieve a particular concentration. For example, commercial pesticides are typically sold as solutions in which the active ingredients are far more concentrated than is appropriate for their application. Before they can be used on crops, the pesticides must be diluted. This is also a very common practice for the preparation of a number of common laboratory reagents (Figure 4).

A simple mathematical relationship can be used to relate the volumes and concentrations of a solution before and after the dilution process. According to the definition of molarity, the molar amount of solute in a solution is equal to the product of the solution’s molarity and its volume in liters:
Expressions like these may be written for a solution before and after it is diluted:
where the subscripts “1” and “2” refer to the solution before and after the dilution, respectively. Since the dilution process does not change the amount of solute in the solution,n1 = n2. Thus, these two equations may be set equal to one another:
This relation is commonly referred to as the dilution equation. Although we derived this equation using molarity as the unit of concentration and liters as the unit of volume, other units of concentration and volume may be used, so long as the units properly cancel per the factor-label method. Reflecting this versatility, the dilution equation is often written in the more general form:
where C and V are concentration and volume, respectively.

Use the simulation to explore the relations between solute amount, solution volume, and concentration and to confirm the dilution equation.
Example 6
Determining the Concentration of a Diluted Solution
If 0.850 L of a 5.00-M solution of copper nitrate, Cu(NO3)2, is diluted to a volume of 1.80 L by the addition of water, what is the molarity of the diluted solution?
Solution
We are given the volume and concentration of a stock solution, V1 and C1, and the volume of the resultant diluted solution, V2. We need to find the concentration of the diluted solution, C2. We thus rearrange the dilution equation in order to isolate C2:
[latex]C_2 = \frac{C_1V_1}{V_2}[/latex]
Since the stock solution is being diluted by more than two-fold (volume is increased from 0.85 L to 1.80 L), we would expect the diluted solution’s concentration to be less than one-half 5 M. We will compare this ballpark estimate to the calculated result to check for any gross errors in computation (for example, such as an improper substitution of the given quantities). Substituting the given values for the terms on the right side of this equation yields:
This result compares well to our ballpark estimate (it’s a bit less than one-half the stock concentration, 5 M).
Check Your Learning
What is the concentration of the solution that results from diluting 25.0 mL of a 2.04-M solution of CH3OH to 500.0 mL?
Answer:
0.102 M CH3OH
Example 7
Volume of a Diluted Solution
What volume of 0.12 M HBr can be prepared from 11 mL (0.011 L) of 0.45 M HBr?
Solution
We are given the volume and concentration of a stock solution, V1 and C1, and the concentration of the resultant diluted solution, C2. We need to find the volume of the diluted solution, V2. We thus rearrange the dilution equation in order to isolate V2:
[latex]V_2 = \frac{C_1V_1}{C_2}[/latex]
Since the diluted concentration (0.12 M) is slightly more than one-fourth the original concentration (0.45 M), we would expect the volume of the diluted solution to be roughly four times the original volume, or around 44 mL. Substituting the given values and solving for the unknown volume yields:
[latex]V_2 = 0.041 \;\text{L}[/latex]
The volume of the 0.12-M solution is 0.041 L (41 mL). The result is reasonable and compares well with our rough estimate.
Check Your Learning
A laboratory experiment calls for 0.125 M HNO3. What volume of 0.125 M HNO3 can be prepared from 0.250 L of 1.88 M HNO3?
Answer:
3.76 L
Example 8
Volume of a Concentrated Solution Needed for Dilution
What volume of 1.59 M KOH is required to prepare 5.00 L of 0.100 M KOH?
Solution
We are given the concentration of a stock solution, C1, and the volume and concentration of the resultant diluted solution, V2 and C2. We need to find the volume of the stock solution, V1. We thus rearrange the dilution equation in order to isolate V1:
[latex]V_2 = \frac{C_2V_2}{C_2}[/latex]
Since the concentration of the diluted solution 0.100 M is roughly one-sixteenth that of the stock solution (1.59 M), we would expect the volume of the stock solution to be about one-sixteenth that of the diluted solution, or around 0.3 liters. Substituting the given values and solving for the unknown volume yields:
[latex]V_1 = 0.314 \;\text{L}[/latex]
Thus, we would need 0.314 L of the 1.59-M solution to prepare the desired solution. This result is consistent with our rough estimate.
Check Your Learning
What volume of a 0.575-M solution of glucose, C6H12O6, can be prepared from 50.00 mL of a 3.00-M glucose solution?
Answer:
0.261 L
Visualizing Different Solutes in Solution
When seeking to understand compounds in solution, we must differentiate between the behavior of covalent compounds and ionic compounds. Assuming the solid dissolves, ionic compounds will dissociate into their ions. In contrast, for covalent compounds, the molecules of the compound remain intact.
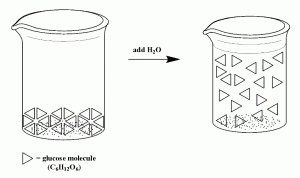
So far in this section, we have been examining solutions of sugar (C6H12O6). If we were to be able to see on the level of molecules, we might see a solution like that below, where a triangle represents a molecule of glucose. While the molecules move around in the solution once they are dissolved in water, the atoms in the molecule do not break apart. The covalent bonds are intact.
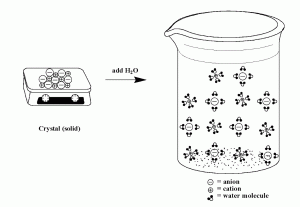
In contrast, when an ionic compound dissolves in solution, the cations and anions are separated (they dissociate) and each ion is surrounded by water molecules. We can depict the dissociation process for a compound like NaCl using the image below:
If we leave out the water molecules (to make it easier to see the ions) below is an example of a sodium sulfate, Na2SO4, solution. The sulfate anions will have a -2 charge and the sodium cations will have a +1 charge.
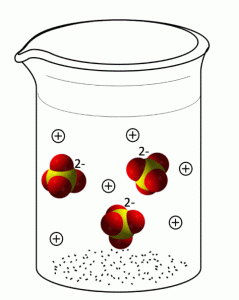
Note that when the ionic compound dissociates, the covalent bonds of any polyatomic ions (like sulfate) remain intact. Also note that the ions in solution are present in the same ratio as in the solid compound. In this case, there are 2 Na+ ions for every 1 SO42- ion.
Example 9
Determining Moles of Ions in Ionic Compounds:
For each of the following, if 1 mole of the compound were to dissolve and dissociate, how many moles of ions would be present in:
a) magnesium chloride?
b) ammonium phosphate?
Solution:
a) The formula for magnesium chloride is MgCl2. It is made of Mg2+ ions and Cl– ions. If a mole of MgCl2 dissociates in solution, we’ll get 1 mole of Mg2+ ions and 2 moles of Cl– ions, for a total of 3 moles of ions.
b) The formula for ammonium phosphate is (NH4)3PO4. It is made of NH4+ ions and PO43- ions. If a mole of (NH4)3PO4 dissociates in solution, we’ll get 3 moles of NH4+ ions and 1 mole of PO43- ions, for a total of 4 moles of ions.
Check your learning:
How many moles of each ion will be produced from 1 mole of ammonium sulfate?
ANSWER:
2 moles of NH4+, 1 mole of SO42-
Because an ionic compound dissociates in solution, we can use our knowledge of molarity to describe the concentration of the compound in solution, OR the concentration of ions in solution.
Example 10
Relating Concentrations of a Compound with Concentrations of its Ions in Solution
If you have a solution that is 0.5 M potassium sulfate, what is the concentration of potassium ions in solution? of sulfate ions in solution?
Solution
To answer the questions above, we want to know how many moles of each ion are present in a liter of solution. We are given that the solution is 0.5 M K2SO4. Given the definition of molarity, we can express the following:
[latex]0.5 \;\text{M K}_2\text{SO}_4 = \frac{0.5 \;\text{mol K}_2\text{SO}_4}{1 \;\text{L}}[/latex]
We can then use the appropriate relationship we know from the formula of potassium sulfate to make the appropriate conversions:
[latex]\frac{0.5 \;\text{mol K}_2\text{SO}_4}{1 \;\text{L}} \times \frac{2 \;\text{mol K}^+}{1 \;\text{mol K}_2\text{SO}_4} = \frac{1 \;\text{mol K}^+}{1 \;\text{L}} = \;\text{1 M K}^+[/latex]
[latex]\frac{0.5 \;\text{mol K}_2\text{SO}_4}{1 \;\text{L}} \times \frac{1 \;\text{mol SO}^{2-}_{4}}{1 \;\text{mol K}_2\text{SO}_4} = \frac{0.5 \;\text{mol SO}^{2-}_{4}}{1 \;\text{L}} = \;\text{0.5 M SO}^{2-}_{4}[/latex]
Does this result make sense? We see that the concentration of potassium ions is twice that of the sulfate ions. Given that when the ions dissociate, there will be twice as many potassium ions present compared to sulfate ion, this result make sense.
Check Your Learning
If you have a 1.3 M solution of ammonium carbonate, (NH4)2CO3, what is the concentration of each ion?
Answer:
2.6 M NH4+, 1.3 M CO32-
Key Concepts and Summary
Solutions are homogeneous mixtures. Many solutions contain one component, called the solvent, in which other components, called solutes, are dissolved. An aqueous solution is one for which the solvent is water. The concentration of a solution is a measure of the relative amount of solute in a given amount of solution. Concentrations may be measured using various units, with one very useful unit being molarity, defined as the number of moles of solute per liter of solution. The solute concentration of a solution may be decreased by adding solvent, a process referred to as dilution. The dilution equation is a simple relation between concentrations and volumes of a solution before and after dilution.
Key Equations
- [latex]M = \frac{\text{mol solute}}{\text{L solution}}[/latex]
- C1V1 = C2V2
Chemistry End of Chapter Exercises
- Explain what changes and what stays the same when 1.00 L of a solution of NaCl is diluted to 1.80 L.
- What information do we need to calculate the molarity of a sulfuric acid solution?
- What does it mean when we say that a 200-mL sample and a 400-mL sample of a solution of salt have the same molarity? In what ways are the two samples identical? In what ways are these two samples different?
- Determine the molarity for each of the following solutions:
(a) 0.444 mol of CoCl2 in 0.654 L of solution
(b) 98.0 g of phosphoric acid, H3PO4, in 1.00 L of solution
(c) 0.2074 g of calcium hydroxide, Ca(OH)2, in 40.00 mL of solution
(d) 10.5 kg of Na2SO4·10H2O in 18.60 L of solution
(e) 7.0 × 10−3 mol of I2 in 100.0 mL of solution
(f) 1.8 × 104 mg of HCl in 0.075 L of solution
- Determine the molarity of each of the following solutions:
(a) 1.457 mol KCl in 1.500 L of solution
(b) 0.515 g of H2SO4 in 1.00 L of solution
(c) 20.54 g of Al(NO3)3 in 1575 mL of solution
(d) 2.76 kg of CuSO4·5H2O in 1.45 L of solution
(e) 0.005653 mol of Br2 in 10.00 mL of solution
(f) 0.000889 g of glycine, C2H5NO2, in 1.05 mL of solution
- Consider this question: What is the mass of the solute in 0.500 L of 0.30 M glucose, C6H12O6, used for intravenous injection?
(a) Outline the steps necessary to answer the question.
(b) Answer the question.
- Consider this question: What is the mass of solute in 200.0 L of a 1.556-M solution of KBr?
(a) Outline the steps necessary to answer the question.
(b) Answer the question.
- Calculate the number of moles and the mass of the solute in each of the following solutions:
(a) 2.00 L of 18.5 M H2SO4, concentrated sulfuric acid
(b) 100.0 mL of 3.8 × 10−5M NaCN, the minimum lethal concentration of sodium cyanide in blood serum
(c) 5.50 L of 13.3 M H2CO, the formaldehyde used to “fix” tissue samples
(d) 325 mL of 1.8 × 10−6M FeSO4, the minimum concentration of iron sulfate detectable by taste in drinking water
- Calculate the number of moles and the mass of the solute in each of the following solutions:
(a) 325 mL of 8.23 × 10−5M KI, a source of iodine in the diet
(b) 75.0 mL of 2.2 × 10−5M H2SO4, a sample of acid rain
(c) 0.2500 L of 0.1135 M K2CrO4, an analytical reagent used in iron assays
(d) 10.5 L of 3.716 M (NH4)2SO4, a liquid fertilizer
- Consider this question: What is the molarity of KMnO4 in a solution of 0.0908 g of KMnO4 in 0.500 L of solution?
(a) Outline the steps necessary to answer the question.
(b) Answer the question.
- Consider this question: What is the molarity of HCl if 35.23 mL of a solution of HCl contain 0.3366 g of HCl?
(a) Outline the steps necessary to answer the question.
(b) Answer the question.
- Calculate the molarity of each of the following solutions:
(a) 0.195 g of cholesterol, C27H46O, in 0.100 L of serum, the average concentration of cholesterol in human serum
(b) 4.25 g of NH3 in 0.500 L of solution, the concentration of NH3 in household ammonia
(c) 1.49 kg of isopropyl alcohol, C3H7OH, in 2.50 L of solution, the concentration of isopropyl alcohol in rubbing alcohol
(d) 0.029 g of I2 in 0.100 L of solution, the solubility of I2 in water at 20 °C
- Calculate the molarity of each of the following solutions:
(a) 293 g HCl in 666 mL of solution, a concentrated HCl solution
(b) 2.026 g FeCl3 in 0.1250 L of a solution used as an unknown in general chemistry laboratories
(c) 0.001 mg Cd2+ in 0.100 L, the maximum permissible concentration of cadmium in drinking water
(d) 0.0079 g C7H5SNO3 in one ounce (29.6 mL), the concentration of saccharin in a diet soft drink.
- There is about 1.0 g of calcium, as Ca2+, in 1.0 L of milk. What is the molarity of Ca2+ in milk?
- What volume of a 1.00-M Fe(NO3)3 solution can be diluted to prepare 1.00 L of a solution with a concentration of 0.250 M?
- If 0.1718 L of a 0.3556-M C3H7OH solution is diluted to a concentration of 0.1222 M, what is the volume of the resulting solution?
- If 4.12 L of a 0.850 M-H3PO4 solution is be diluted to a volume of 10.00 L, what is the concentration of the resulting solution?
- What volume of a 0.33-M C12H22O11 solution can be diluted to prepare 25 mL of a solution with a concentration of 0.025 M?
- What is the concentration of the NaCl solution that results when 0.150 L of a 0.556-M solution is allowed to evaporate until the volume is reduced to 0.105 L?
- What is the molarity of the diluted solution when each of the following solutions is diluted to the given final volume?
(a) 1.00 L of a 0.250-M solution of Fe(NO3)3 is diluted to a final volume of 2.00 L
(b) 0.5000 L of a 0.1222-M solution of C3H7OH is diluted to a final volume of 1.250 L
(c) 2.35 L of a 0.350-M solution of H3PO4 is diluted to a final volume of 4.00 L
(d) 22.50 mL of a 0.025-M solution of C12H22O11 is diluted to 100.0 mL
- What is the final concentration of the solution produced when 225.5 mL of a 0.09988-M solution of Na2CO3 is allowed to evaporate until the solution volume is reduced to 45.00 mL?
- A 2.00-L bottle of a solution of concentrated HCl was purchased for the general chemistry laboratory. The solution contained 868.8 g of HCl. What is the molarity of the solution?
- An experiment in a general chemistry laboratory calls for a 2.00-M solution of HCl. How many mL of 11.9 M HCl would be required to make 250 mL of 2.00 M HCl?
- What volume of a 0.20-M K2SO4 solution contains 57 g of K2SO4?
- The US Environmental Protection Agency (EPA) places limits on the quantities of toxic substances that may be discharged into the sewer system. Limits have been established for a variety of substances, including hexavalent chromium, which is limited to 0.50 mg/L. If an industry is discharging hexavalent chromium as potassium dichromate (K2Cr2O7), what is the maximum permissible molarity of that substance?
Glossary
- aqueous solution
- solution for which water is the solvent
- concentrated
- qualitative term for a solution containing solute at a relatively high concentration
- concentration
- quantitative measure of the relative amounts of solute and solvent present in a solution
- dilute
- qualitative term for a solution containing solute at a relatively low concentration
- dilution
- process of adding solvent to a solution in order to lower the concentration of solutes
- dissolved
- describes the process by which solute components are dispersed in a solvent
- molarity (M)
- unit of concentration, defined as the number of moles of solute dissolved in 1 liter of solution
- solute
- solution component present in a concentration less than that of the solvent
- solvent
- solution component present in a concentration that is higher relative to other components
Solutions
Answers to Chemistry End of Chapter Exercises
2. We need to know the number of moles of sulfuric acid dissolved in the solution and the volume of the solution.
4. (a) 0.679 M;
(b) 1.00 M;
(c) 0.06998 M;
(d) 1.75 M;
(e) 0.070 M;
(f) 6.6 M
6. (a) determine the number of moles of glucose in 0.500 L of solution; determine the molar mass of glucose; determine the mass of glucose from the number of moles and its molar mass; (b) 27 g
8. (a) 37.0 mol H2SO4;
3.63 × 103 g H2SO4;
(b) 3.8 × 10−6 mol NaCN;
1.9 × 10−4 g NaCN;
(c) 73.2 mol H2CO;
2.20 kg H2CO;
(d) 5.9 × 10−7 mol FeSO4;
8.9 × 10−5 g FeSO4
10. (a) Determine the molar mass of KMnO4; determine the number of moles of KMnO4 in the solution; from the number of moles and the volume of solution, determine the molarity; (b) 1.15 × 10−3M
12. (a) 5.04 × 10−3M;
(b) 0.499 M;
(c) 9.92 M;
(d) 1.1 × 10−3M
14. 0.025 M
16. 0.5000 L
18. 1.9 mL
20. (a) 0.125 M;
(b) 0.04888 M;
(c) 0.206 M;
(e) 0.0056 M
22. 11.9 M
24. 1.6 L

