108 19.4 Potential, Free Energy, and Equilibrium
Learning Objectives
- Relate cell potentials to free energy changes
- Use the Nernst equation to determine cell potentials at nonstandard conditions
- Perform calculations that involve converting between cell potentials, free energy changes, and equilibrium constants
We will now extend electrochemistry by determining the relationship between [latex]E_{\text{cell}}^{\circ}[/latex] and the thermodynamics quantities such as ΔG° (Gibbs free energy) and K (the equilibrium constant). In galvanic cells, chemical energy is converted into electrical energy, which can do work. The electrical work is the product of the charge transferred multiplied by the potential difference (voltage):
The charge on 1 mole of electrons is given by Faraday’s constant (F)
In this equation, n is the number of moles of electrons for the balanced oxidation-reduction reaction. The measured cell potential is the maximum potential the cell can produce and is related to the electrical work (wele) by
The negative sign for the work indicates that the electrical work is done by the system (the galvanic cell) on the surroundings. In an earlier chapter, the free energy was defined as the energy that was available to do work. In particular, the change in free energy was defined in terms of the maximum work (wmax), which, for electrochemical systems, is wele.
We can verify the signs are correct when we realize that n and F are positive constants and that galvanic cells, which have positive cell potentials, involve spontaneous reactions. Thus, spontaneous reactions, which have ΔG < 0, must have Ecell > 0. If all the reactants and products are in their standard states, this becomes
This provides a way to relate standard cell potentials to equilibrium constants, since
Most of the time, the electrochemical reactions are run at standard temperature (298.15 K). Collecting terms at this temperature yields
where n is the number of moles of electrons. For historical reasons, the logarithm in equations involving cell potentials is often expressed using base 10 logarithms (log), which changes the constant by a factor of 2.303:
Thus, if ΔG°, K, or [latex]E_{\text{cell}}^{\circ}[/latex] is known or can be calculated, the other two quantities can be readily determined. The relationships are shown graphically in Figure 1.
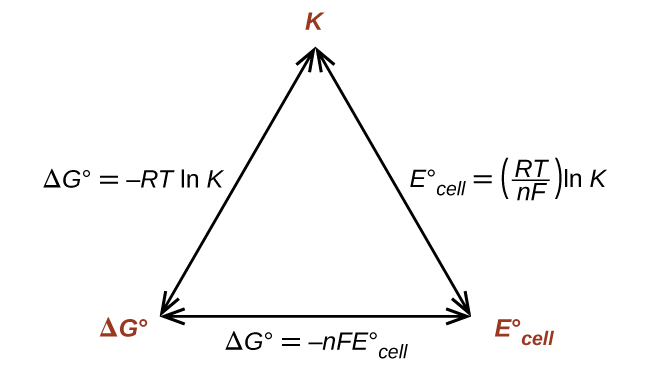
Given any one of the quantities, the other two can be calculated.
Example 1
Equilibrium Constants, Standard Cell Potentials, and Standard Free Energy Changes
What is the standard free energy change and equilibrium constant for the following reaction at 25 °C?
Solution
The reaction involves an oxidation-reduction reaction, so the standard cell potential can be calculated using the data in Appendix L.
[latex]\begin{array}{lr @{{}\longrightarrow{}} ll} \text{anode (oxidation):} & \text{Fe}(s) & \longrightarrow \text{Fe}^{2+}(aq)\;+\;2\text{e}^{-} \\[0.5em] \text{cathode (reduction):} & 2\;\times\;(\text{Ag}^{+}(aq)\;+\;\text{e}^{-} & \longrightarrow\text{Ag}(s)) \\[0.5em]\end{array}\\[0.5em][/latex]
[latex]\begin{array}{lrl @{{}={}} ll} E_{\text{anode}}^{\circ} = & E_{\text{Fe}^{2+}/\text{Fe}}^{\circ} = & -0.447\;\text{V} \\[0.5em] E_{\text{cathode}}^{\circ} = & E_{\text{Ag}^{+}/\text{Ag}}^{\circ} = & 0.7996 \;\text{V} \\[0.5em]\end{array}\\[0.5em][/latex]
[latex]E_{\text{cell}}^{\circ} = E_{\text{cathode}}^{\circ}\;-\;E_{\text{anode}}^{\circ} = 0.7996 \;\text{V} - (-0.447\;\text{V}) = +1.247\;\text{V}[/latex]
Remember that the cell potential for the cathode is not multiplied by two when determining the standard cell potential. With n = 2, the equilibrium constant is then
The two equilibrium constants differ slightly due to rounding in the constants 0.0257 V and 0.0592 V. The standard free energy is then
Check your answer: A positive standard cell potential means a spontaneous reaction, so the standard free energy change should be negative, and an equilibrium constant should be >1.
Check Your Learning
What is the standard free energy change and the equilibrium constant for the following reaction at room temperature? Is the reaction spontaneous?
Answer:
[latex]E_{\text{cell}}^{\circ} = +0.291\;\text{V}\\[0.5em]{\Delta}G^{\circ} = -56.2\;\frac{\text{kJ}}{\text{mol}}[/latex]
K = 6.8 × 109
Spontaneous
Now that the connection has been made between the free energy and cell potentials, nonstandard concentrations follow. Recall that
where Q is the reaction quotient (see the chapter on equilibrium fundamentals). Converting to cell potentials:
This is the Nernst equation. At standard temperature (298.15 K), it is possible to write the above equations as
If the temperature is not 273.15 K, it is necessary to recalculate the value of the constant. With the Nernst equation, it is possible to calculate the cell potential at nonstandard conditions. This adjustment is necessary because potentials determined under different conditions will have different values.
Example 2
Cell Potentials at Nonstandard Conditions
Consider the following reaction at room temperature:
Is the process spontaneous?
Solution
There are two ways to solve the problem. If the thermodynamic information in Appendix G were available, you could calculate the free energy change. If the free energy change is negative, the process is spontaneous. The other approach, which we will use, requires information like that given in Appendix L. Using those data, the cell potential can be determined. If the cell potential is positive, the process is spontaneous. Collecting information from Appendix L and the problem,
[latex]\begin{array}{lr @{{}\longrightarrow{}} ll} \text{anode (oxidation):} & \text{Co}(s) & \longrightarrow \text{Co}^{2+}(aq)\;+\;2\text{e}^{-} \\[0.5em] \text{cathode (reduction):} & \text{Fe}^{2+}(aq)\;+\;\text{2e}^{-} & \longrightarrow\text{Fe}(s) \\[0.5em]\end{array}\\[0.5em][/latex]
[latex]\begin{array}{lrl @{{}={}} ll} E_{\text{anode}}^{\circ} = & E_{\text{Co}^{2+}/\text{Co}}^{\circ} = & -0.28\;\text{V} \\[0.5em] E_{\text{cathode}}^{\circ} = & E_{\text{Fe}^{2+}/\text{Fe}}^{\circ} = & -0.477 \;\text{V} \\[0.5em]\end{array}\\[0.5em][/latex]
[latex]E_{\text{cell}}^{\circ} = E_{\text{cathode}}^{\circ}\;-\;E_{\text{anode}}^{\circ} = -0.447 \;\text{V} - (-0.28\;\text{V}) = -0.17\;\text{V}[/latex]
The process is not spontaneous under standard conditions. Using the Nernst equation and the concentrations stated in the problem and n = 2,
The process is (still) nonspontaneous.
Check Your Learning
What is the cell potential for the following reaction at room temperature?
What are the values of n and Q for the overall reaction? Is the reaction spontaneous under these conditions?
Answer:
n = 6; Q = 1440; Ecell = +1.97 V, spontaneous.
Finally, we will take a brief look at a special type of cell called a concentration cell. In a concentration cell, the electrodes are the same material and the half-cells differ only in concentration. Since one or both compartments is not standard, the cell potentials will be unequal; therefore, there will be a potential difference, which can be determined with the aid of the Nernst equation.
Example 3
Concentration Cells
What is the cell potential of the concentration cell described by
Solution
From the information given:
[latex]\begin{array}{lrl @{{}={}} ll} E_{\text{anode}}^{\circ} = & E_{\text{Zn}^{2+}/\text{Zn, 0.10 M}}^{\circ} = & -0.7618\;\text{V} \\[0.5em] E_{\text{cathode}}^{\circ} = & E_{\text{Zn}^{2+}/\text{Zn, 0.50 M}}^{\circ} = & -0.7618 \;\text{V} \\[0.5em]\end{array}\\[0.5em][/latex]
[latex]E_{\text{cell}}^{\circ} = E_{\text{cathode}}^{\circ}\;-\;E_{\text{anode}}^{\circ} = -0.7618 \;\text{V} - (-0.7618\;\text{V}) = 0.0000\;\text{V}[/latex]
The standard cell potential is zero because the anode and cathode involve the same reaction; only the concentration of Zn2+ changes.
Substituting the concentrations into the Nernst equation,
and the process is spontaneous at these conditions.
Check your answer: In a concentration cell, the standard cell potential will always be zero. To get a positive cell potential (spontaneous process) the reaction quotient Q must be <1. Q < 1 in this case, so the process is spontaneous.
Check Your Learning
What value of Q for the previous concentration cell would result in a voltage of 0.10 V? If the concentration of zinc ion at the cathode was 0.50 M, what was the concentration at the anode?
Answer:
Q = 0.00042; [Zn2+]cat = 2.1 × 10−4M.
Key Concepts and Summary
Electrical work (wele) is the negative of the product of the total charge (Q) and the cell potential (Ecell). The total charge can be calculated as the number of moles of electrons (n) times the Faraday constant (F = 96,485 C/mol e−). Electrical work is the maximum work that the system can produce and so is equal to the change in free energy. Thus, anything that can be done with or to a free energy change can also be done to or with a cell potential. The Nernst equation relates the cell potential at nonstandard conditions to the logarithm of the reaction quotient. Concentration cells exploit this relationship and produce a positive cell potential using half-cells that differ only in the concentration of their solutes.
Key Equations
- [latex]E_{\text{cell}}^{\circ} = \frac{RT}{nF}\;\text{ln}\;K \\[0.5em][/latex]
- [latex]E_{\text{cell}}^{\circ} = \frac{0.0257\;\text{V}}{n}\;\text{ln}\;K = \frac{0.0592\;\text{V}}{n}\;\text{log}\;K\;(\text{at}\;298.15\;K) \\[0.5em][/latex]
- [latex]E_{\text{cell}} = E_{\text{cell}}^{\circ}\;-\;\frac{RT}{nF}\;\text{ln}\;Q\;(\text{Nernst equation}) \\[0.5em][/latex]
- [latex]E_{\text{cell}} = E_{\text{cell}}^{\circ}\;-\;\frac{0.0257\;\text{V}}{n}\;\text{ln}\;Q = \frac{0.0592\;\text{V}}{n}\;\text{log}\;Q\;(\text{at}\;298.15\;K) \\[0.5em][/latex]
- [latex]{\Delta}G = -nFE_{\text{cell}} \\[0.5em][/latex]
- [latex]{\Delta}G^{\circ} = -nFE_{\text{cell}}^{\circ} \\[0.5em][/latex]
- [latex]w_{\text{ele}} = w_{\text{max}} = -nFE_{\text{cell}} \\[0.5em][/latex]
Chemistry End of Chapter Exercises
- For the standard cell potentials given here, determine the ΔG° for the cell in kJ.
(a) 0.000 V, n = 2
(b) +0.434 V, n = 2
(c) −2.439 V, n = 1
- For the ΔG° values given here, determine the standard cell potential for the cell.
(a) 12 kJ/mol, n = 3
(b) −45 kJ/mol, n = 1
- Determine the standard cell potential and the cell potential under the stated conditions for the electrochemical reactions described here. State whether each is spontaneous or nonspontaneous under each set of conditions at 298.15 K.
(a) [latex]\text{Hg}(l)\;+\;\text{S}^{2-}(aq\text{, }0.10\;M)\;+\;2\text{Ag}^{+}(aq\text{, }0.25\;M)\;{\longrightarrow}\;2\text{Ag}(s)\;+\;\text{HgS}(s)[/latex]
(b) The galvanic cell made from a half-cell consisting of an aluminum electrode in 0.015 M aluminum nitrate solution and a half-cell consisting of a nickel electrode in 0.25 M nickel(II) nitrate solution.
(c) The cell made of a half-cell in which 1.0 M aqueous bromine is oxidized to 0.11 M bromide ion and a half-cell in which aluminum ion at 0.023 M is reduced to aluminum metal. Assume the standard reduction potential for Br2(l) is the same as that of Br2(aq).
- Determine ΔG and ΔG° for each of the reactions in the previous problem.
- Use the data in Appendix L to determine the equilibrium constant for the following reactions. Assume 298.15 K if no temperature is given.
(a) [latex]\text{AgCl}(s)\;{\leftrightharpoons}\;\text{Ag}^{+}(aq)\;+\;\text{Cl}^{-}(aq)[/latex]
(b) [latex]\text{CdS}(s)\;{\leftrightharpoons}\;\text{Cd}^{2+}(aq)\;+\;\text{S}^{2-}(aq)\;\;\;\;\;\;\;\text{at}\;377\;\text{K}[/latex]
(c) [latex]\text{Hg}^{2+}(aq)\;+\;4\text{Br}^{-}(aq)\;{\leftrightharpoons}\;[\text{HgBr}_4]^{2-}(aq)[/latex]
(d) [latex]\text{H}_2\text{O}(l)\;{\leftrightharpoons}\;\text{H}^{+}(aq)\;+\;\text{OH}^{-}(aq)\;\;\;\;\;\;\;\text{at}\;25^{\circ}\;\text{C}[/latex]
Glossary
- concentration cell
- galvanic cell in which the two half-cells are the same except for the concentration of the solutes; spontaneous when the overall reaction is the dilution of the solute
- electrical work (wele)
- negative of total charge times the cell potential; equal to wmax for the system, and so equals the free energy change (ΔG)
- Faraday’s constant (F)
- charge on 1 mol of electrons; F = 96,485 C/mol e−
- Nernst equation
- equation that relates the logarithm of the reaction quotient (Q) to nonstandard cell potentials; can be used to relate equilibrium constants to standard cell potentials
Solutions
Answers to Chemistry End of Chapter Exercises
1. (a) 0 kJ/mol; (b) −83.7 kJ/mol; (c) +235.3 kJ/mol
3. (a) standard cell potential: 1.50 V, spontaneous; cell potential under stated conditions: 1.43 V, spontaneous; (b) standard cell potential: 1.405 V, spontaneous; cell potential under stated conditions: 1.423 V, spontaneous; (c) standard cell potential: −2.749 V, nonspontaneous; cell potential under stated conditions: −2.757 V, nonspontaneous
5. (a) 1.7 × 10−10; (b) 2.6 × 10−21; (c) 8.9 × 1019; (d) 1.0 × 10−14

